Vibrational spectroscopies are integral in analyzing some of the most fundamentally important processes in physical chemistry: molecular vibrations. While there are many different experimental techniques used to analyze those vibrations, most are variations of the “Big Two,” FTIR and Raman scattering spectroscopies. This article aims to analyze the benefits and drawbacks of each of the “Big Two,” with the hope that it helps one choose the correct technique for one’s research interests.
1. What are the “Big Two?”
Raman spectroscopy relies on inelastic scattering phenomenon that probes the molecular vibration. Where FTIR will use a broadband IR source, Raman spectroscopy typically uses a narrow-band, monochromatic light source in order to excite the vibrations of the molecule in your sample.
Fourier Transform Infrared Spectroscopy (FTIR) is a simple absorption measurement where the detector measures the absorbance of infrared radiation by the sample. Each sample will absorb different amounts of each frequency resulting in a “chemical fingerprint” that is the FTIR spectrum.
2. Selection Rules: What molecular vibrations are being probed?
Imagining a molecular bond vibration with the traditional ball and spring model (Figure 1a) and its resultant harmonic oscillator depiction (Figure 1b) allows us to calculate the selection rules for vibrational transitions to be Dn = ± 1. However, that does not tell the whole story. In order to truly differentiate FTIR from Raman Spectroscopy, we must think about it on a molecular level.
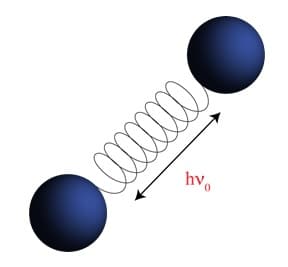
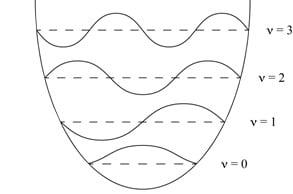
Figure 1: A. Ball and spring model used to approximate molecular vibrations. B. Depiction of the calculated energy levels of a molecular vibration using the Harmonic Oscillator approximation.
Without diving to deeply into the quantum mechanics (more info can be found HERE1), Dn = ± 1 is only partially correct as it concerns the direct absorption of infrared radiation. In the case of Raman spectroscopy, we are considering inelastic scattering of radiation (typically) in the visible range. In scattering phenomenon, upon interaction with an atom (or molecule), the light’s oscillating electric field (E) will induce and oscillating polarization (P) that is governed by Equation 1:

Here E and P are depicted as vectors, and a is the polarizability of the molecule in question. This induced oscillation will produce radiation that is the same frequency as the original electric field in a process called elastic scattering. Equation 1 is certainly true for spherical molecules; of course, in real world applications we are rarely dealing with perfect spheres, and so we can imagine a scenario where a molecular vibration alters the polarizability of that molecule, resulting in a Polarization field that is different than that of the incident electric field. This process is called inelastic scattering, and it is this quantum mechanical idea that is exploited by Raman spectroscopy. Thus, we have a separate selection rule for probing molecular vibrations using Raman spectroscopy: the molecular vibration must result in a change in polarizability of the molecule.
It is important to note, that FTIR also has an additional selection rule, the origin of which we will not dive into here because it involves a bit more quantum mechanics and complex mathematical analysis (an in-depth derivation can be found HERE1). That selection rule is stated as such: the molecular vibration must result in a change in the dipole moment of the molecule.
In order to truly differentiate the two, we must consider what is actually happening to the molecule when various vibrational modes are excited. Let us model this using a simple system, CO2 (Figure 2).
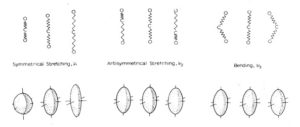
Figure 2: Depiction of the three (unique) vibrations associated with CO2 (top), and the polarization ellipsoids of those modes (bottom). Figure adapted from the UC Berkeley Chem 125 Lab Manual.
Observing the polarization ellipsoids of n2 and n3, we can see that the antisymmetric stretch and bending modes of CO2 result in a simple translation of the of the polarizability but no real change. Only n1 results in a change in the polarizability of the molecule, and so it is the only Raman active vibrational mode. Incidentally, there is no change in the dipole moment for a n1 stretch, so this mode is can NOT be detected using FTIR.
3. Comparing and contrasting FTIR and Raman
Raman
- Measures the relative frequencies at which a sample scatters radiation.
- Sensitive to homo-nuclear and non-polar bonds. For example, Raman can distinguish between C-C, C=C, and CºC bonds.
- Because the incident radiation source is typically in the visible range, (as is the scattered light), any colorless cuvette can be used. This means that aqueous samples are quite easily analyzed with high sensitivity.
- Again, because the scattered light being detected is in the visible wavelength range, the detectors are quite inexpensive. More on Raman Spectrometer Options
- Very amenable to microscopy experiments.
- The scattering phenomenon means one angular dependence of a sample can be analyzed, e.g. nanocrystals or chiral molecules.
- Due to thermal distribution of the ground state vibrational levels, Raman is particularly sensitive to temperature. See Fundamentals of Raman
FTIR
- Measures the absolute frequency at which a sample absorbs radiation
- Sensitive to hetero-nuclear functional group vibrations, polar bonds, etc.
- Requires very specific containment apparatus in order to detect certain samples. For example, most cuvettes (quartz, plastic, etc) will absorb all infrared light in stretching regions 2500 cm-1 and above. This means that without an expensive (and delicate) CaF2 (or something similar) sandwich cell, studying aqueous samples is impossible.
- Detectors can be quite expensive, as the materials which detect IR radiation (typically InGaAs or Ge) are very expensive.
- Not very amenable to microscopy techniques, as the FTIR microscopy method has constraints on sample
More info about StellarNet Raman Spectrometer Systems
(1) Harris, D. C.; Bertolucci, M. D. Symmetry and Spectroscopy: An Introduction to Vibrational and Electronic Spectroscopy; Dover Publications Inc.: New York, USA, 1990.

By Tony Rizzuto, PhD – Physical Chemistry
StellarNet Technical Staff Writer
UC Berkeley Chemistry





