The principle of absorption spectroscopy is to measure how much light is absorbed by the sample. As seen in the absorbance entry, this can be accomplished by measuring the intensity of the light before and after the sample. The Beer-Lambert law describes how the absorbance is related to the experimental parameters:
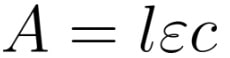
where A is the absorbance, l is the path length through the sample (e.g. the length of the cuvette holding the sample), is the molar attenuation coefficient (also called the molar extinction coefficient or the molar absorptivity), and c is the concentration of the species of interest in the sample. The molar attenuation coefficient depends on wavelength and can be used to identify species in the sample, so measuring an absorption spectrum over many wavelengths tells you what substance(s) is (are) present and in what amounts. Absorbance is additive, so when N species are present, the total absorbance is:
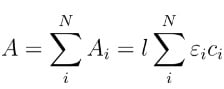
To determine the composition of a mixture with N species, you can measure absorbance at N wavelengths and solve the set of N linear equations. The ChemWiz ID application included in the SpectraWiz software can also be used to assign spectra of unknown species.
(813) 855-8687
ContactUs@StellarNet.us





