Tis the Season!

A Diamond is Forever?
You’re about to ask the biggest question of your life, on one knee, in front of her entire family, on Christmas Eve. You’re nervous. Your mind starts to go to the dark places… Are you sure that sparkly rock is a diamond? What if it’s only cubic zirconia? Or moissanite? Does the chemical structure of the stone really matter, as long as your love is real?
Well, StellarNet can’t help you answer if your love is real or will last forever, but we can tell you that at room temperature and pressure, a diamond will not last forever; eventually thermodynamics will convert the diamond to it’s preferred graphite structure. Lucky for you, the kinetics is relatively slow, and it would take tens of billions of years for the change to happen. So, if tens of billions of years seems like forever to you, then, yes, for practical purposes, your diamond will last forever.
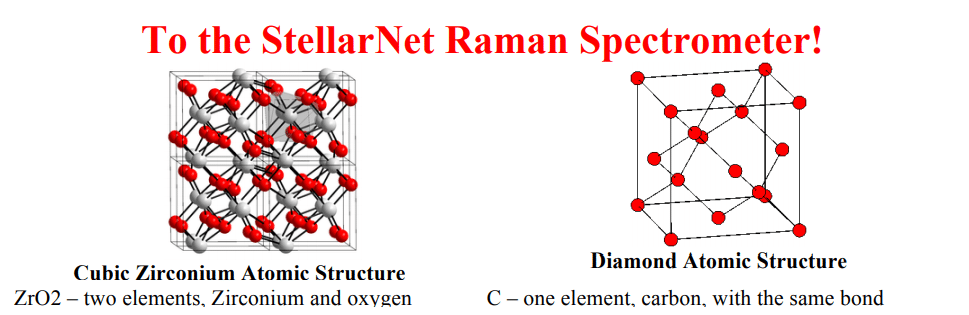
StellarNet can also help you determine the composition of that sparkly rock. Diamond and cubic zirconia stone are both clear and sparkly. But one is composed entirely of carbon atoms arranged in a tetrahedral-diamond structure, and one is a compound of zirconium and oxygen, ZrO2. To the naked eye, both are beautiful… but to a StellarNet Raman Spectrometer, they’re quite different.
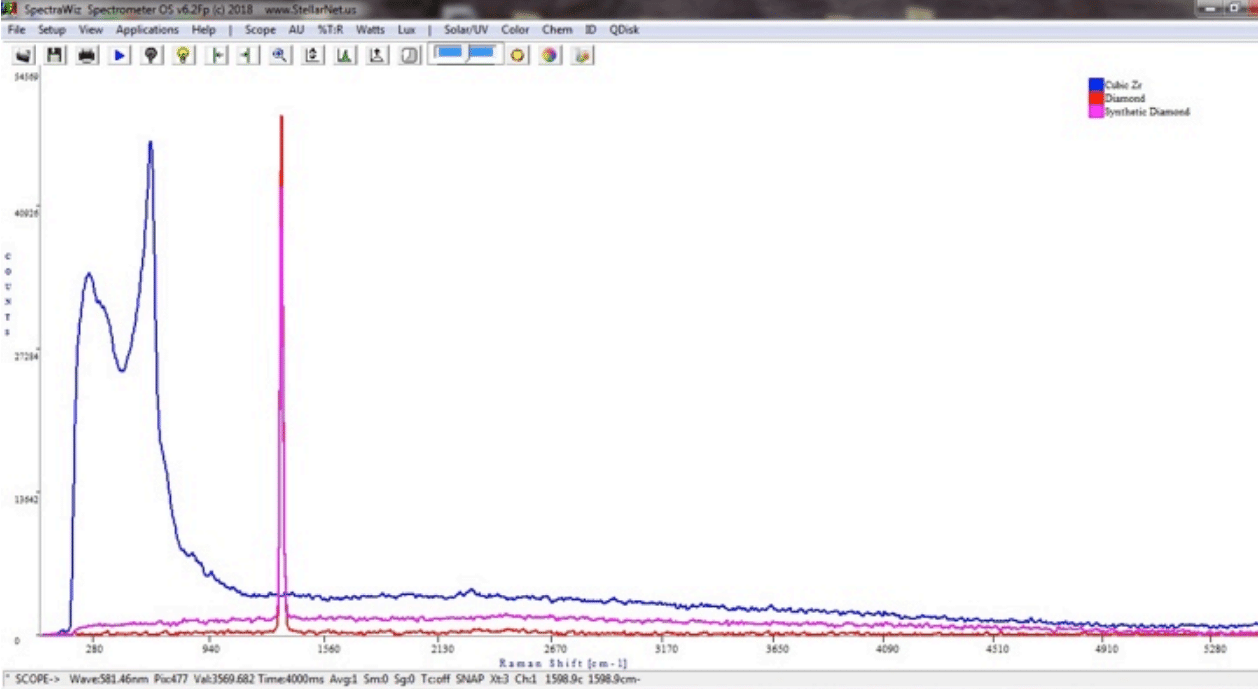
Raman is a great tool. Diamond’s Raman signature is easy to identify due to the fact that all the carbon atoms in a diamond are equally and tetrahedral bound to one another covalently. That means that the same vibrational property between every atom in the lattice is the same, and that is reflected in the Raman Spectroscopy data shown. The diamond Raman signature and the cubic zirconia Raman signature are completely different. So, now you know you have a diamond… but was it dug out of the ground, or created in a lab…?
FUN FACT:
You can become a diamond! You’re made of carbon; diamonds are made of carbon. So, can your carbon atoms be rearranged to become a diamond? Yes! (StellarNet suggests that you wait until you don’t need your body anymore before you contact…)

Lifegem.com is a company that turns ashes into diamonds. From their website: “What is a LifeGem? The LifeGem is a certified, high-quality diamond created from the ashes of your loved one as a memorial to their unique and wonderful life. The LifeGem diamond can also be made from the carbon in a lock of hair.

532nm Raman Spectra of a Diamond (both natural and synthetic) compared to Cubic Zirconia using the StellarSCOPE, Raman-HR-TEC-532 spectrometer, and Lab Laser. You can clearly see the 1332cm-1 characteristic peak for carbon in diamond.
Are we so Different? Natural versus Man-Made Diamond
StellarNet’s customers are interested in a more challenging question: the characteristic differences between natural out-of-the-ground diamonds and lab-created man-made diamonds. Would the love-of-your-life care if the diamond is natural or man-made? We can’t answer that; however, the jewelry industry is quite interested in that answer, as more and more lab-grown diamonds come onto the market.
StellarNet customers are working on identifying natural and synthetic diamonds, and even creating unique spectral fingerprints for each. Recently published research (2018 – https://link.springer.com/article/10.1007/s11182-018 1422-6 ) shows that by using the optical absorption and cathodoluminescence spectra of diamond, it might be possible to clearly identify natural diamonds from high-pressure diamonds from diamonds created by another lab process called chemical vapor deposition.
What about the other Gemstones?
StellarNet customers all over the world use our spectrometers to identify and quantify gemstones. Using fluorescence, different rocks, minerals, and gemstones often show phenomenally different responses to UV light. Shown here, rocks in white light, and the same rocks under ultraviolet light.


Mining Gemstones
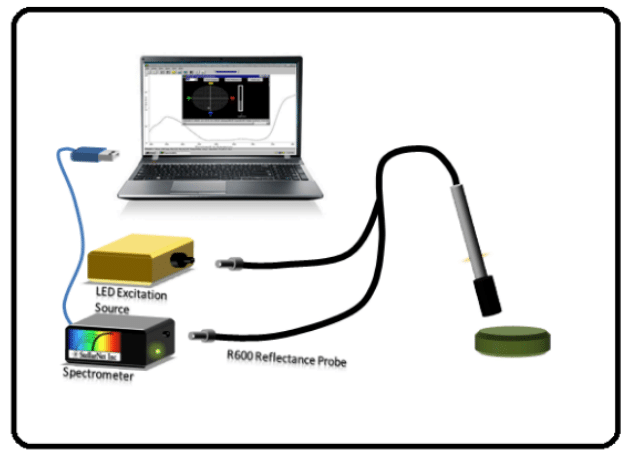
Customers in the mining industry are using StellarNet’s ultra-rugged spectrometers in the field to identify the purity of emeralds, as shown here in this customer sample; the emerald crystal can be seen in the reflected facet. This is an image from the field of a customer using StellarNet’s spectrometers to analyze the rock in a mining operation. The setup used is similar to the diagram here – the customer is using a TE cooled spectrometer, a fluorescence probe and a LED excitation source – the only difference is instead of a laptop, the customer is holding a handheld tablet.
Inspired by Gemstones
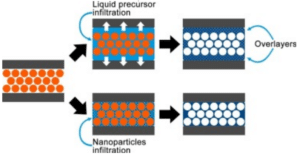
Other StellarNet customers are using spectroscopy to study materials with similar properties to gemstones. A team in Argentina just published, “Characterization of titania inverse opals prepared by two distinct infiltration approaches” in which they prepare “an opal template, so-called because of their structural similitude with the precious gemstones (https://www.sciencedirect.com/science/article/pii/S0025540817330301 ) In this paper, they study specular reflectance spectroscopy using a StellarNet SILVER-Nova.
About StellarNet
StellarNet, Inc. manufactures precision fiber optic spectrometers for portable and multi-channel industrial applications, which enable low cost spectroscopy solutions. Our expertise in electro-optics, software design, and applications development, provides unmatched price performance in the global instrumentation market.