Every week, it seems like another friend or family member is diagnosed with cancer. The American Cancer Society predicts that 1,688,780 people will be diagnosed with cancer and 600,920 people will die from cancer in the US in 2017. This problem isn’t limited to the US, either. The World Cancer Research Fund International estimates that there were 14.1 million cancer cases worldwide in 2012. Aside from the obvious humanitarian aspect of this problem, it’s also a huge economic burden. The American Cancer Society estimates that cancer related health care costs were $87.8 billion in 2014.
Clearly this is a worldwide problem. Why is a solution so difficult? The word “cancer” actually includes many diseases, each with their own set of characteristics and treatment. They have a common mechanism, though: somehow, normal cells start behaving erratically and grow out of control, preventing other cells from performing their function. This presents two main problems: One, cancer cells end up being very similar to normal cells as far as treatments go. This means finding treatments that are specific to cancer cells is difficult. Two, the cause of cells turning cancerous is complex and difficult to pinpoint for prevention or targeted treatment.
Still, cancer researchers persist. Here is a collection of studies by StellarNet’s customers.
Treatments Using Low Temperature Plasma Devices
The Keidar group at George Washington University, as well as the Yang group at the Chinese Academy of Sciences, have both been working with plasma devices for treating cancer. The specific devices are low temperature, meaning they can be used in biological systems, and operate at atmospheric pressure, meaning expensive vacuum equipment is not necessary.
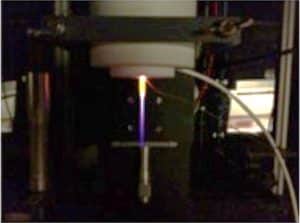
A plasma needle device. Adapted from Figure 1 in Ref. [8].
The discharge, formed with Ar and O gases, showed peaks for NO, OH, N2, N, Ar, and O. However, the NO and N2 peaks disappear inside the media. This suggests that the nitrogen radicals produced by the discharge are absorbed by the media and that the oxygen radicals are the ones that diffuse to the cells and cause ablation.
Breast cancer cells. Figure by Ewa Krawczyk, https://visualsonline.cancer.gov/details.cfm?imageid=10623.
The Keidar group has experimented with two types of plasma devices. The first is actually a combination of plasma with a static magnetic field [3]. The authors used a StellarNet spectrometer and probe to measure the emission spectrum of the plasma with and without the static magnetic field; the spectra were identical, proving that the field did not alter the plasma. They then treated breast cancer MDA-MB-231 cells with four treatments:
- direct plasma treatment (cells were present when the plasma was applied) only
- direct plasma treatment + static magnetic field
- indirect plasma treatment (plasma was applied to the media and then cells added) only indirect plasma treatment + static magnetic field
- indirect plasma treatment + static magnetic field
Treatment 2 performed the best; it was 8.5 times more likely to kill cancer cells than normal cells! The second device was an atmospheric pressure microdischarge plasma device [4]. It created self organized patterns on the interface between the plasma and the medium. At low current and voltage (Type I), the plasma created only one filament at the interface (similar to the needle), but high current and voltage (Type IV) produced many filaments. They again used StellarNet products to collect the emission spectra, which were similar. Both types were able to decrease the survival rate of breast cancer MDA-MB-231 cells and glioblastoma U87 cells, but had more of an effect on the glioblastoma cells. The high current stage actually cause the reactive oxygen species to decrease over time, which is the opposite behavior observed for most other plasma devices
Gold Nanoparticles for Photothermal Ablation
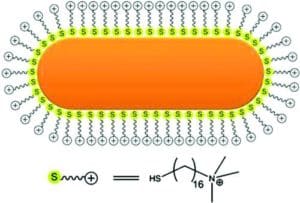
Reprinted with permission from Ref. [9]. Copyright 2011 American Chemical Society.

Reprinted with permission from Ref. [6]. Copyright 2016 American Chemical Society.
Skin Lesion Classification
Before cancer can be treated, it needs to be diagnosed. Melanoma is one type of cancer that is tricky to diagnose. Currently, dermatologists have to rely on their own visual inspection to determine if skill lesions should be biopsied. Technology can help streamline this process and possibly help avoid biopsies altogether. Safi et. al. used reflectance spectroscopy along with machine learning to come up with a model for predicting if a lesion is harmful or not [7]. They used a BLACK-Comet-SR spectrometer with a 12W tungsten lamp and reflectance probe to measure the reflectance spectra of legions. A principal components analysis (PCA) was used to decrease the dimensionality of the dataset and then it was input into a support vector machine (SVM) algorithm. This algorithm is good for finding decision boundaries between classes when said boundaries are likely to be nonlinear. This is achieved by transforming the nonlinear data with a kernel to a new vector space where the boundary is linear. Then the calculated boundary is transformed back to the original space. The authors used cross validation to determine that using radial basis functions as the kernel and using the top 4 or 5 principle components gave the best accuracy; both combinations were able to classify the lesions with 94.9% accuracy.
Cancer is an extremely complex problem, but researchers are extremely dedicated. With a world full of scientists looking for a solution, it’s only a matter of time before a complete solution is found.
This article is shared in loving memory of Michele Nordstrom, wife of Tyler Nordstrom, our senior optics engineer; who lost her battle this week.
References
1. Huang J, Chen W, Li H, Wang X-Q, Lv G-H, Khohsa ML, et al. Deactivation of A549 cancer cells in vitro by a dielectric barrier discharge plasma needle. J Appl Phys. 2011;109: 053305.
2. Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008;93: 021502.
3. Cheng X, Rajjoub K, Shashurin A, Yan D, Sherman JH, Bian K, et al. Enhancing cold atmospheric plasma treatment of cancer cells by static magnetic field. Bioelectromagnetics. 2017;38: 53–62.
4. Chen Z, Zhang S, Levchenko I, Beilis II, Keidar M. In vitro Demonstration of Cancer Inhibiting Properties from Stratified Self-Organized Micro-Discharge Plasma-Liquid Interface [Internet]. arXiv. 2017. Available: https://arxiv.org/abs/1701.01655
5. Rossi F, Ratto F, Pini R. Laser Activated Gold Nanorods for the Photothermal Treatment of Cancer. COMSOL; Available: https://www.comsol.com/paper/laser-activated-goldnanorods-for-the-photothermal-treatment-of-cancer-13260
6. Zhu G, Liu J-T, Wang Y, Zhang D, Guo Y, Tasciotti E, et al. In Situ Reductive Synthesis of Structural Supported Gold Nanorods in Porous Silicon Particles for Multifunctional Nanovectors. ACS Appl Mater Interfaces. 2016;8: 11881–11891.
7. Safi A, Castaneda V, Lasser T, Navab N. Skin Lesions Classification with Optical Spectroscopy. In: Liao H, Edwards PJ “eddie,” Pan X, Fan Y, Yang G-Z, editors. Medical Imaging and Augmented Reality. Berlin, Heidelberg: Springer Berlin Heidelberg; 2010. pp. 411–418.
8. Virard F, Cousty S, Cambus J-P, Valentin A, Kémoun P, Clément F. Cold Atmospheric Plasma Induces a Predominantly Necrotic Cell Death via the Microenvironment. PLoS One. 2015;10: e0133120.
9. Bao Y, Vigderman L, Zubarev ER, Jiang C. Robust multilayer thin films containing cationic thiol-functionaliz

By Debra McCaffrey, PhD
StellarNet Technical Staff Writer
UC Berkeley Chemistry





