In the previous experiments, you were introduced to visible and fluorescence spectroscopy. You learned that different molecules absorb and emit different wavelengths of light and that you can characterize molecules based on these patterns. Now you will learn about another spectroscopic technique: Raman spectroscopy. You will use a combination of computer simulations and Raman
spectroscopy to study chemical bonding.
use a computer simulation of springs to model the properties of single, double, and triple covalent bonds and bonds with different types of atoms (different masses). You will explore what factors impact how bonds oscillate. Then, you will then explore the relationship between bond vibrations and Raman spectroscopy in hexane, 1-hexene, and 1-heptyne..
Chemical Bonding
There are many different models for describing covalent chemical bonds and the motion of molecules. Molecules have three types of motion: translation, rotational, and vibrational. Translation motion is when molecules move from one position to another. Rotational motion entire molecule rotating or the internal parts of the molecule rotating with respect to one another. Vibrational motion is when bonds between atoms within a molecule move. There are many different types of molecular vibrations possible with such colorful names as symmetric and antisymmetric stretching, scissoring, rocking, wagging and twisting. Modeling a covalent bond as a spring helps illustrate the vibration of bonds in a molecule. When an atom is displaced from its equilibrium position in a molecule (e.g. when it stretches or wags or twists), it is subject to a restoring force. A spring follows this same pattern, which is called Hooke’s law. Thus, a chemical bond can be modeled as a spring that has weights (atoms) attached to its two ends. This system has a natural vibrational frequency which depends on the masses of the weights and the stiffness of the spring
Bonds are always vibrating from thermal energy present in the surroundings. Light (electromagnetic radiation) can interact with these molecular vibrations in certain predictable ways. Raman spectroscopy uses the scattering of light from molecular vibrations can be used to characterize molecules.
Raman Spectroscopy
In the previous experiments, you were introduced to visible and fluorescence spectroscopy. You learned that different molecules absorb and emit different wavelengths of light and that you can characterize molecules based on these patterns. Now you are going to use another spectroscopic technique: Raman spectroscopy.
Raman spectroscopy exposes a sample to a single wavelength of light, usually from a laser in the visible, infrared, or ultraviolet range. The laser light is then scattered by the sample. Scattering simply means that the light particles (photons) are forced to deviate from their straight path by some interaction with the sample. In Raman spectroscopy, the laser light interacts with molecular vibrations resulting in the energy of the laser photons being shifted up or down (e.g. Raman shift). The shift in energy provides useful information about the vibrational modes in the system and can be used to identify the type of bonds present in the compound.
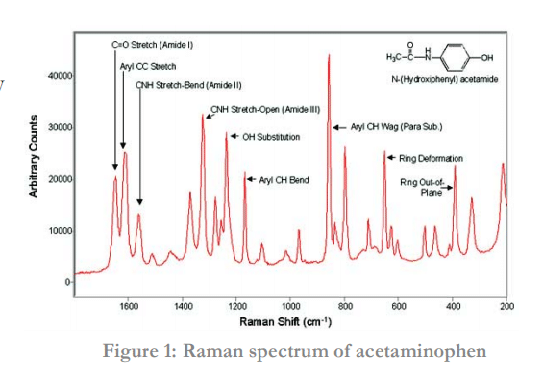
A Raman spectrum is unique to a material and thus is an excellent technique for identifying compounds. A Raman spectrum consists of a range of features, each associated with a vibrational mode (see Figure 1). Each type of bond (e.g. C-H, C-C, C=C) has unique vibrational modes, which leads to specific interactions with light and thus produce different Raman shifts (see Table 1). This information can be used to identify molecules and study chemical bonding.