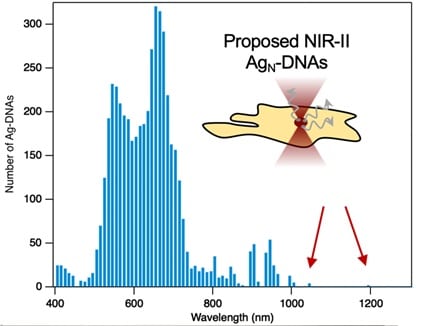
Congratulations to our second-place winner Professor Stacy Copp, Ph.D. Candidate Anna Gonzalez Rosell, and their team at the University of California, Irvine. They are using biomolecular engineering to develop NIR bio-labels that is within a class of fluorophores. The DNA sequence determines the atomic size and creates a fluorescence color. Professor Copp and team are working on the development of AG-DNA that have longer wavelengths and thus are in the NIR range of the spectrum. This is advantageous because the longer wavelengths that these nano-particles are emitting in the NIR range are more stable and less toxic so are very beneficial for use in biomedical imaging. Their goal is to use our NIR DWARF-Star spectrometer to develop biocompatible and environmentally responsible fluorophores that have emission peaks between 900-1,200nm. This development would be a major breakthrough for non-invasive deep-tissue imaging and could be used in numerous medical applications and specialties.
Image of fluorescent Ag-DNAs on a well-plate that have been excited using a UV transilluminator
Image of fluorescent Ag-DNAs on a well-plate that have been excited using a UV transilluminator
Abstract
Near infrared (NIR) light penetrates much deeper into biological tissues than visible light, making NIR imaging a promising approach for noninvasive biomedical imaging. However, deep tissue imaging has been hindered by a lack of small, bright, and non-toxic fluorophores, especially in the NIR-II window above 1000 nm, where biological tissues are transparent up to centimeters in depth. We are developing new NIR-II biolabels through biomolecular engineering of an emerging class of fluorophores called DNA-stabilized silver nanoclusters (Ag-DNAs). Ag-DNAs are unique or their “genomic” properties: the sequence of the templating DNA molecule tunes the nanocluster’s atomic size and fluorescence color. This tunability has been used to synthesize a range of Ag-DNAs with emission peaks spanning 450-1000 nm. We are developing chemistry-informed machine learning models to guide the discovery of new Ag-DNAs with even longer emission wavelengths in the NIR-II window, and with the high stability and low toxicity required for biomedical imaging. Our models have led to our recent discovery of Ag-DNAs with peak emission up to 1,200 nm (in review, ACS Nano). We aim to develop these nanoclusters as biocompatible and environmentally responsible fluorophores for deep tissue imaging, as compared to toxic NIR-II emitters such as carbon nanotubes and quantum dots. The StellarNet DWARF-Star NIR spectrometer will allow us to dramatically advance our research, expanding our spectral characterization capabilities above 900 nm and enabling us to develop a library of NIR-II biolabels that will enable new frontiers in noninvasive deep tissue imaging, for applications ranging from cancer research and detection to cardiovascular monitoring.