Xuejing Shen, Tao Sun, Lei Yang, Alexey Krasnoslobodtsev, Renat Sabirianov, Michael Sealy, Wai-Ning Mei, Zhanjun Wu & Li Tan
Abstract
With the rapid iteration of portable electronics and electric vehicles, developing high-capacity batteries with ultra-fast charging capability has become a holy grail. Here we report rechargeable aluminum-ion batteries capable of reaching a high specific capacity of 200 mAh g−1. When liquid metal is further used to lower the energy barrier from the anode, fastest charging rate of 104 C (duration of 0.35 s to reach a full capacity) and 500% more specific capacity under high-rate conditions are achieved. Phase boundaries from the active anode are believed to encourage a high-flux charge transfer through the electric double layers. As a result, cationic layers inside the electric double layers responded with a swift change in molecular conformation, but anionic layers adopted a polymer-like configuration to facilitate the change in composition.
Introduction
Fast charging is the key feature for portable electronics and electric vehicles which has ignited vigorous research activities. For energy storage platforms that rely on reversible redox reactions, the reduction in charging time from hours to minutes has already become a reality. A typical example can be found in a non-lithium platform, i.e., Al-ion batteries. Over the past five years, it has quickly captured the fame of exceptional rate in both charging and discharging. The adoption of a pure Al as the electrode provides significant merits such as low cost, nonflammability, and high capacity. In addition, a stable Al electrode-electrolyte interface removes the complexity from an interphase layer that is commonly seen in lithium or lithium-ion systems. As such, long lasting performance with several tens of thousands of reversible charging and discharging has been demonstrated.
Can we further reduce the charging time from minutes to fractions of a second while keeping most of the capacity? Read publication…
Raman Measurements
Raman measurements were performed using a Raman spectrometer configured in transmission mode on the Olympus IX71 inverted optical microscope. An oil immersion Olympus objective lens with 100X magnification and 1.4 NA (UPLSAPO) was utilized for focusing the laser on the surface of anode before collecting the Raman signal. Glass coverslips windows were created in a home-made sealed chamber. The chambers were placed on the stage equipped with an x-y-positioning piezoelectric controller. Two experimental setups were utilized: (1) Ntegra-Spectra (NT–MDT, Moscow, Russia) and (2) Raman-HR-TEC (StellarNet, Inc., Tampa, USA). (1) Ntegra-Spectra was utilized for initial detection of reaction species on the anode. Ntegra-Spectra detects the Raman signal using an Andor–CCD camera cooled to −60 °C and optically coupled with both the Raman spectrometer and inverted microscope. A diode laser with λ = 532 nm and a nominal power of 100 mW was used for excitation (LaserExportCo, Ltd, Moscow, Russia). (2) Raman-HR-TEC (StellarNet, Inc., Tampa, FL, USA) was utilized for automated fast collection of spectra at various charging densities. The spectrometer is coupled to both the inverted microscope and the laser (λ = 647 nm and a nominal power of 150 mW) using the Raman Probe—the fiber optics cable (StellarNet, Inc.) which integrates both excitation and collection cables. Home-built LabView (National Instruments, Austin, TX, USA) interface in “time series” mode was utilized allowing for collection of spectra without delays at various current densities especially suitable for signal collection at fast rates. Each spectrum was collected for a total of 5 s acquisition time and background corrected for both instruments. Go to StellarNet Raman options…
Results
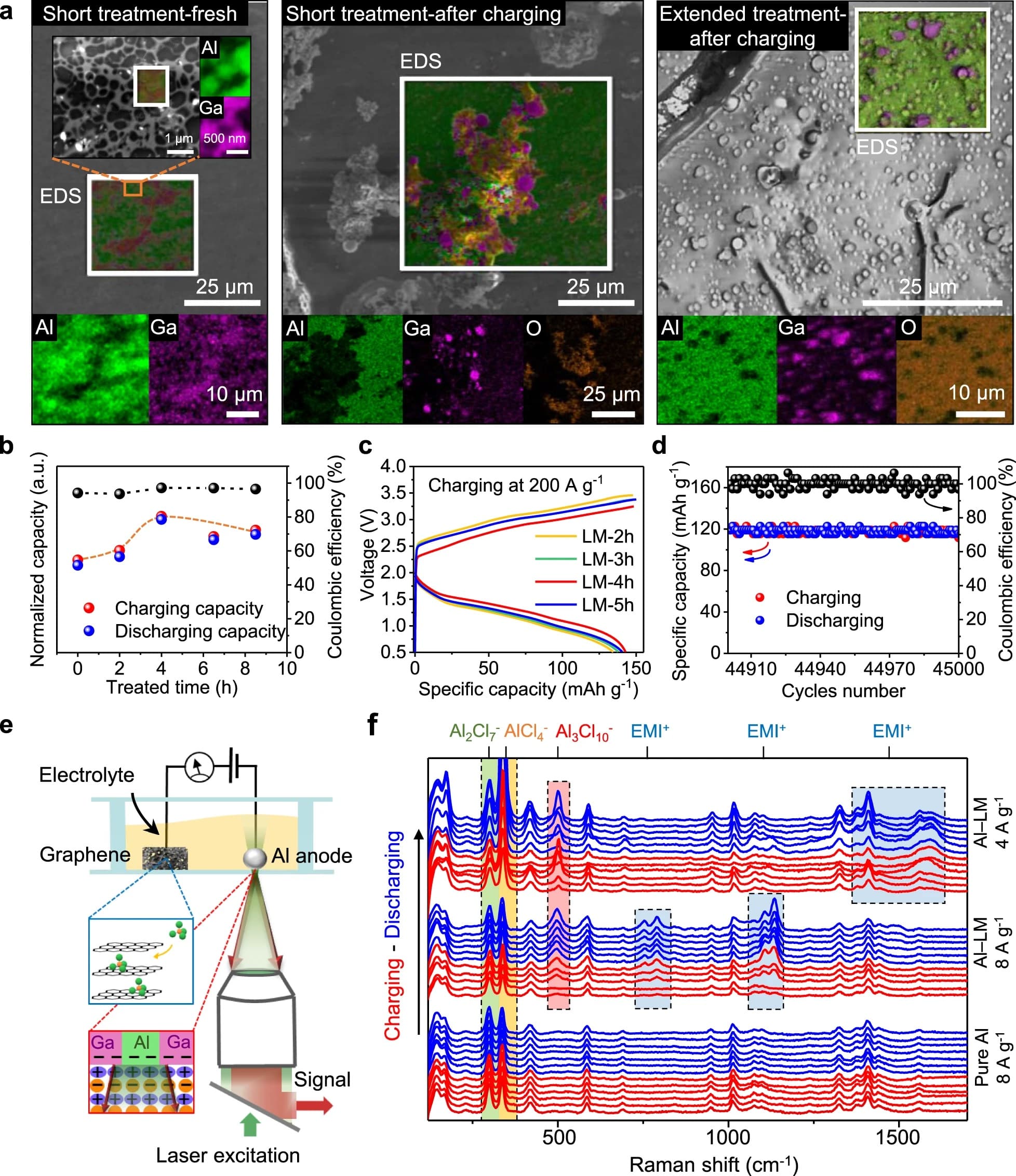
Al-ion batteries earned their fame by using an organic cation-based electrolyte, similar to those cases in lithium and lithium-ion batteries. Different from metal salts in water, cations here do not have any metal element; therefore, they don’t directly participate in redox reactions. Instead, the metal ions exist as anions or as negatively charged metal complexes. Preparation of the electrolyte is straightforward: mixing imidazolium chloride (EMI+Cl−) (solid) and anhydrous powder of AlCl3 produces an ionic liquid (eutectic mixture). Three major ions have been reported in this electrolyte, i.e., Al mono-complex (AlCl4−), Al duo-complex (Al2Cl7−), and the organic cation (EMI+)5,15. When this electrolyte is placed inside an Al-ion battery, the Al electrode will be biased negatively and carbon electrode positively for charging. As a result, electrons from Al will jump over to the Al duo-complex and reduce it to a mono-complex, depositing fresh Al(0) over the Al electrode. On the carbon side, no new products will form. Rather, the Al mono-complex will adsorb on positively charged carbon surfaces. When batteries are allowed to discharge, Al (anode) will be oxidized but the carbon (cathode) reduced.
We used a three-dimensional (3D) network of graphene as the cathode to promote charge capacity, along with pure Al as the anode. Figure 1a shows the network structure of our graphene, where the carbon-growth on a nickel foam was handled inside a chemical vapor deposition (CVD) chamber (see Supplementary Fig. 1). Later removal of the nickel template requested acid dissolution, solvent rinsing, and drying. We found that the graphene cathode can exhibit smaller redox potentials in cyclic voltammogram (Fig. 1b) only when the drying step is handled using supercritical CO2. Shifted peaks in the voltammogram suggest higher affinity for anions (AlCl4−) to bind to the surface (Fig. 1a-middle); an open and continuous network would then allow for a reliable desorption (Supplementary Figs. 2 and 3). Seemingly, the graphene cathode acts as an open pocket by holding anions (AlCl4−) during the charging process. When these anions bind to the graphene (positively biased while charging), three carbon-chloride bonds (Fig. 1a-middle) could form, rendering a robust “holding” of the Al mono-complexes. As strong bonding lowers energy of the system, we hypothesize that further cleavage of these bonds would be energetically costly, making discharge prohibitive under a high rate. Read publication…





