StellarNet’s President, Jason Pierce, explains some of the practical differences of Raman and NIR spectroscopy, which include the inability for NIR to measure aqueous samples, the surface area of detection, portability, specificity, SERS, and fluorescence. A demonstration of common nutraceuticals, supplements, and ingredients also shows how fluorescence can be problematic for botanical samples yet work well with NIR or Raman 1064nm. See all StellarNet Raman and NIR systems
Although Raman and NIR spectroscopy share some similarities – there are distinct advantages and disadvantages to each measurement technique. Any analytical lab should have both techniques available but this may not be an option for everyone. Here are some main ideas and suggestions that should guide your choice between Raman and NIR.
If you need to measure aqueous samples or samples with a significant amount of water in them – NIR is NOT the way to go. NIR measures the absorbance of the vibrational modes of a sample. NIR has large water absorbances that prohibit anything with water inside of it to not be measurable. In drier samples, these large absorbance bands make NIR great for moisture detection. With aqueous or samples with lots of water – Raman is your best choice.
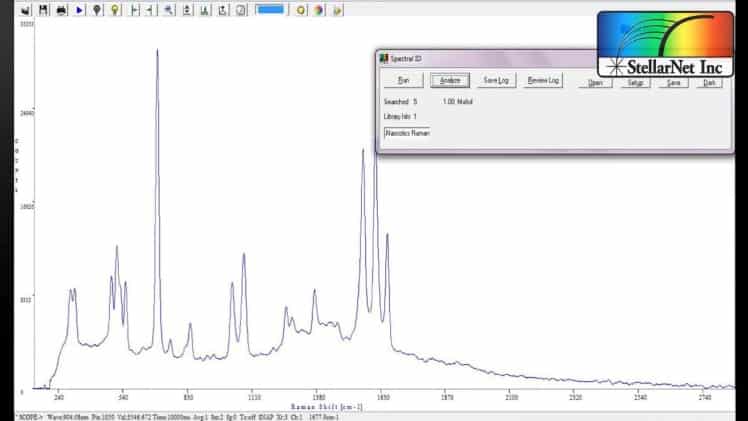
In a Raman spectrum, you have sharp emission peaks which are representative of a molecule. When you have mixtures of molecules you can easily identify each molecule by these sharp emission lines. This means that you don’t have to have all of your samples or mixtures of samples in your library database in order to get spectral matches. Alternatively, NIR spectra is characterized by broad reflectance bands of the vibrational overtones of your molecules.
These vibrational bands are great for quantifying samples. With the addition of chemometrics, NIR is a great tool for quantitative analysis. The StellarCASE-NIR comes with starter calibrations for a variety of different sample types – allowing the user to do composition analysis.
A well-known challenge with Raman spectroscopy is fluorescence. This occurs when you illuminate your sample with the Raman laser and you cause an excited state of your molecule and it emits light in all directions. This typically saturates the detector so you can’t read your Raman peaks.
Now for the million-dollar questions…
What samples fluoresce and which samples do not fluoresce? How do you know? When should I go to NIR and when should I utilize Raman?
You can first check out our StellarRAM libraries page. The StellarRAM has a few different libraries that are included with the instrument. These databases include hazardous materials, narcotics, chemicals, minerals, and more!
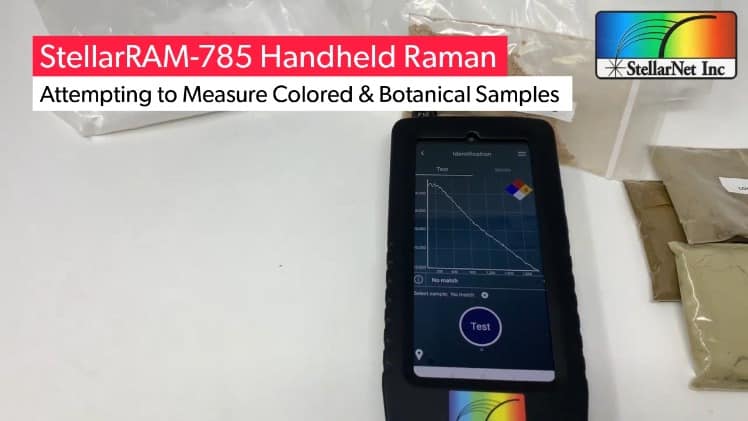
Typically, the rule of thumb is if you have organic materials or materials with impurities they will tend to cause a lot more fluorescence than samples without. Colored samples can also cause additional fluorescence as well as higher power lasers.
For example, a 405nm UV Raman system will cause more fluorescence than a 532nm green or 785nm red laser. This is why we offer a 1064nm NIR Raman system.
The NIR laser reduces fluorescent and allows you to measure a lot more samples. But – there are drawbacks to the 1064nm laser. Specifically, we need to use a NIR detector which is more expensive and these detectors require thermoelectric cooling. This requires a larger package, more power consumption, and you have to wait longer because you are using a weaker laser.
A common example where both NIR and Raman works is nutraceuticals. But – there are a variety of different nutraceuticals that are made of organic substances and ground leaves so they are not necessarily pure extracts. These types of samples can cause fluorescence and may not be a good fit for a 785nm excitation with Raman.
As complementary techniques, NIR and Raman are great analytical tools for your lab. If you have questions about what technique is right for you send us an email: ContactUs@StellarNet.us and one of our Application Scientists will help you decide!