
Overview:
Last month’s technical note titled “Raman Fundamentals for Miniature Spectrometers” introduced the physical principals of Raman spectroscopy and typical experimental set-ups. One crucial topic not discussed in that article was the relatively low probability of spontaneous Raman scattering, which limits the sensitivity of the technique. The exact probability of spontaneous Raman scattering, defined as a molecule’s Raman cross-section, is highly dependent on both the sample and the excitation wavelength, but it is generally acceptable approximate it as 10-7. As a result, Raman spectroscopy typically is not capable of measuring samples with concentrations less than 1%. For bulk samples, the benefits of Raman spectroscopy typically out-weigh its limited sensitivity, but when performing trace analysis spontaneous Raman generally does not provide the required sensitivity.
There are several analytical techniques which can induce stimulated Raman scattering, greatly enhancing the probability of Raman scattering, but most of these methods require ultra-fast tunable lasers and microscopy set-ups making them impractical for most users. By contrast, surface-enhanced Raman spectroscopy (SERS), utilizes surface plasmons to enhance Raman scattering by several orders of magnitude, without requiring different equipment than traditional Raman spectroscopy. This technical note will serve as the first publication in a two-part series on SERS and will mainly focus on the fundamentals of the technique, with particular emphasis the impact of the different nanostructures used for SERS and experimental considerations.
The Role of Surface Plasmons and Nanostructures:
Surface plasmons are generated when nanoparticles or films with nanoscale (20–300 nm) roughness, made of noble metals, typically gold or silver, are subjected to large electric fields. These nanoparticles can also be deposited into other porous materials such as paper (P-SERS) which can provide a type of hybrid structure often referred to as a flexible substrate. While an exhaustive analysis of the physics of surface plasmons is beyond the scope of this paper, it is essential to understand that this complex interaction between the metal, plasmon, and an analyte can cause both an electromagnetic (104 – 107) and a chemical enhancement (10 – 102) to the Raman scattering efficiency. The primary enhancement factor is from the electromagnetic field generated by the surface plasmon. Therefore the geometry of the nanostructures is critical in determining the surface plasmons’ wavelengths and the enhancement factor for a given excitation wavelength. While the nanostructure determines the exact value of the peak enhancement wavelength, the metal itself determines the range over which plasmons can be generated. Typically silver substrates tend to have higher performance when excited by laser sources in the green or red region of the spectrum, and gold substrates tend to perform better when excited in the near infrared.
Figure 1 below was taken from a review article on SERS titled, “Fundamentals and applications of SERS-based bioanalytical sensing,” by Kahraman Et. al. demonstrates a wide variety of SERS substrates including nanoparticles, solid substrates, and flexible substrates each having their unique advantages and disadvantages.
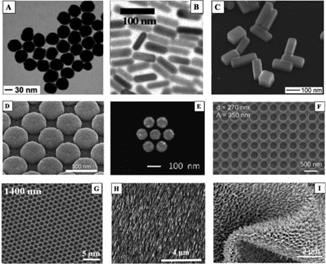
Figure (1) SEM images of various SERS substrates as demonstrated in “Fundamentals and applications of SERS-based bioanalytical sensing,” by Kahraman Et. al.
For trace detection, it is often helpful to functionalized SERS substrates and nanoparticles with antibodies or other molecules which trap a particular analyte of interest while allowing the remainder of the sample to be rinsed off. Widely used in biomedical diagnostics, this process can reduce the “noise” associated with complex background matrix, but it is important to point out that the addition of these relatively long capture molecules increases the spacing between the substrate and analyte resulting in a decrease in enhancement. This process also adds cost and complexity and therefore should only be used when necessary.
Typical SERS Experimental Set-up:
Unlike traditional Raman spectroscopy where solid samples are easily measurable, SERS requires that the sample is either dissolved in a solution with the colloidal nanoparticles or dissolved in solution and pipetted onto the substrate. In either case, once the sample is deposited onto the substrate or in solution with the nanoparticles, illuminating the sample with a focused laser provides the excitation source for both the Raman scattering and plasmon generation. Figure 2 shows three different methods for preparing a sample for use with P-SERS substrates, and the comparison between the Raman spectrum and the SERS spectrum for Morphine. In sample preparation (a) the substrate is dipped into the solution, in (b) traditional micro-pipetting techniques are used, and (c) utilizes a swabbing technique.
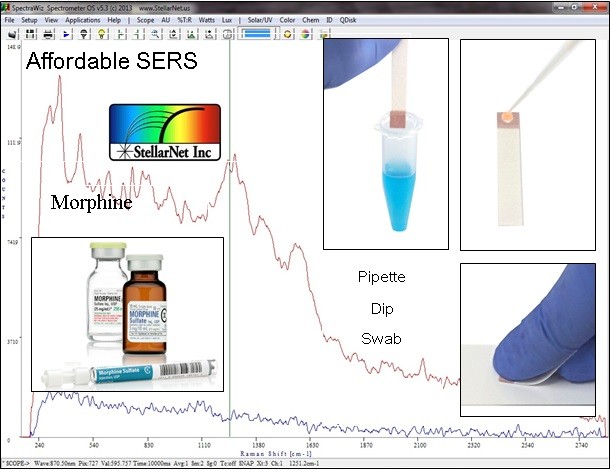
Figure 2: P-SERS spectrum of Morphine showing three different sample preparation techniques (a) dip, (b), pipette and (c) swab.
While the instrumentation required for SERS is the same as in traditional Raman spectroscopy (laser, probe, and spectrometer), there are a few additional considerations to ensure optimal performance. It may sound counter-intuitive, but when taking a SERS measurement, it is generally best to use a multi-mode excitation laser instead of a single-mode laser. Three main factors contribute to this reason, and the first and most important is to maximize the number of nanoparticles or nanostructures within the laser spot. Exciting more “nano-edges” within the substrate will increase the generation of surface plasmons and improve the enhancement factor; therefore single-mode lasers which typically focus to a sub-10 mm spot will often lead to lower enhancement than a multimode laser which will typically focus to a 100mm spot. The second reason why a single-mode laser is not preferable for SERS is the spatial distribution of the analyte. Solid substrates and colloidal solutions can both experience inhomogeneity, but most striking is the “coffee ring” effect observed when the solvent in the pipetted sample evaporates leaving a ring of high concentration around the perimeter of the spot. Lastly, many SERS substrates including P-SERS are strong absorbers at the excitation wavelength. Therefore, a tightly focused single-mode laser will have a much higher chance of thermally damaging the sample than a multimode laser of equivalent power.
Conclusion:
After reading this article, one should now have a working knowledge of the advantages and disadvantages of SERS as a method for detecting low concentrations of a specific analyte of interest. This article provided a brief introduction to the role of surface plasmons in the enhancement process, as described a variety of nanostructures which can be used to generate them – lastly, a general overview of common experimental considerations we presented. The second part in this series will delve deeper into several application examples and compare and contrast a variety of SERS technologies.





