Alcoholic beverages seem like a simple concept at first: take something sugary, throw in some yeast, and ta da! The sugars are fermented into ethanol. But the reality is more complex, even for mass produced products like Budweiser and Charles Shaw. For example, wine characteristics are often determined by when the grapes are picked. The longer grapes remain on the vine, the more tannins ripen and the more sugar forms. For late harvest wines and ice wines, which are expected to be sweet, this is not a problem. However, for red wines where tannis are desirable, the wine is usually dry, meaning all sugar has been fermented to ethanol. Too much sugar means a high alcohol content for dry wines. This is just a small part of how grapes are grown, called viticulture. This doesn’t cover making wine from said grapes, let alone touching on other types of alcoholic beverages. Here’s a sampling of how StellarNet customers are contributing to these complex processes.
Beer
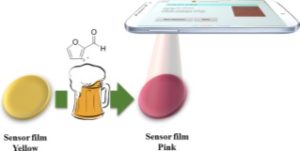
Reprinted with permission from Ref. [1]. Copyright 2016 American Chemical Society
Wine
 Speaking of grape harvesting, Michael Fadock worked on a spectroscopic method to determine the appropriate time to harvest grapes [2]. Currently, grapes are transported to a lab to measure the sugar content (in units of °Brix), pH, and titratable acids. When these quantities reach a certain threshold, the grapes are harvested. However, there is usually a delay between when the grapes are
Speaking of grape harvesting, Michael Fadock worked on a spectroscopic method to determine the appropriate time to harvest grapes [2]. Currently, grapes are transported to a lab to measure the sugar content (in units of °Brix), pH, and titratable acids. When these quantities reach a certain threshold, the grapes are harvested. However, there is usually a delay between when the grapes are
sent to the lab and when the results are available. Fadock combined reflection spectroscopy with partial least squares to predict the three quantities without having to measure them, using a BLACK-Comet spectrometer to collect the spectra. He was able to predict the °Brix with good accuracy, but pH and titratable acid predictions were less accurate.
 Another important property of grapes is where they are grown, such as Napa or Bordeaux. This is captured by the protected designation of origin (PDO), which is a target of forfeiters. Current techniques for determining PDO are expensive, time-consuming, and destroy large amounts of sample. Moncayo et. al. combined laser-induced breakdown spectroscopy (LIBS) with neural networks to create a new technique for confirming PDO [3]. Using a BLUE-Wave spectrometer, they measured the Mg, Ca, K, and Na spectral lines of wines to input into the neural network model. This produced predictions that were >98% accurate for new wine samples, including some that didn’t have a PDO.
Another important property of grapes is where they are grown, such as Napa or Bordeaux. This is captured by the protected designation of origin (PDO), which is a target of forfeiters. Current techniques for determining PDO are expensive, time-consuming, and destroy large amounts of sample. Moncayo et. al. combined laser-induced breakdown spectroscopy (LIBS) with neural networks to create a new technique for confirming PDO [3]. Using a BLUE-Wave spectrometer, they measured the Mg, Ca, K, and Na spectral lines of wines to input into the neural network model. This produced predictions that were >98% accurate for new wine samples, including some that didn’t have a PDO.
The Manufacturing Process
Because ethanol is so volatile, a lot of the ethanol produced in fermentation evaporates into the air. This can create a number of hazards, including fire, health, and environmental. Therefore, it is imperative to have accurate ethanol gas sensors. Parthibavarman et. al.  synthesized a Co-doped SnO2 material for ethanol sensing [4]. They used a white light source and BLUE-Wave spectrometer to characterize the material through UV-vis reflectance, among other techniques. They discovered that the band gap decreases with increasing Co content. As a sensor, the material was sensitive to ethanol, showed a quick response time when ethanol gas was introduced, and showed reversibility when ethanol gas was turned off.
synthesized a Co-doped SnO2 material for ethanol sensing [4]. They used a white light source and BLUE-Wave spectrometer to characterize the material through UV-vis reflectance, among other techniques. They discovered that the band gap decreases with increasing Co content. As a sensor, the material was sensitive to ethanol, showed a quick response time when ethanol gas was introduced, and showed reversibility when ethanol gas was turned off.
Aside from environmental hazards of ethanol gas, there are also waste products from the fermentation process to consider. Han et. al. investigated a new method for the remediation of waste, using ethanol as a model organic compound [5]. Generally, an iron-based Fenton reaction with hydrogen peroxide is used to oxidize organic waste, but the reaction creates waste of its own. A magnesium-based catalyst produces water as the only byproduct, leading to a cleaner reaction. The researchers demonstrated the reaction with Mn3O4 instead of an iron compound. They monitored the hydrogen peroxide through UV-vis spectroscopy, collected by a BLUE-Wave spectrometer. The magnesium catalyst was not as efficient as the iron catalyst, but it was indeed cleaner. The reaction mechanism was also different.
A lot of effort and research goes into a glass of alcohol. These are just a few of the ways that spectroscopy can participate in the process. I’ll raise my glass to that!
References
- Rico-Yuste A, González-Vallejo V, Benito-Peña E, de Las Casas Engel T, Orellana G, Moreno-Bondi MC. Furfural Determination with Disposable Polymer Films and Smartphone-Based Colorimetry for Beer Freshness Assessment. Anal Chem. 2016;88: 3959–3966.
- Fadock M. Non-Destructive VIS/NIR Reflectance Spectrometry for Red Wine Grape Analysis [Internet]. Brown R, editor. Master’s, University of Guelph. 2011. Available: http://hdl.handle.net/10214/2827
- Moncayo S, Rosales JD, Izquierdo-Hornillos R, Anzano J, Caceres JO. Classification of red wine based on its protected designation of origin (PDO) using Laser-induced Breakdown Spectroscopy (LIBS). Talanta. 2016;158: 185–191.
- Parthibavarman M, Renganathan B, Sastikumar D. Development of high sensitivity ethanol gas sensor based on Co-doped SnO2 nanoparticles by microwave irradiation technique. Curr Appl Phys. 2013;13: 1537–1544.
- Han Y-F, Chen F, Zhong Z, Ramesh K, Chen L, Jian D, et al. Complete oxidation of low concentration ethanol in aqueous solution with H2O2 on nanosized Mn3O4/SBA-15 catalyst. Chem Eng J. 2007;134: 276–281.

By Debra McCaffrey, PhD
StellarNet Technical Staff Writer
UC Berkeley Chemistry





