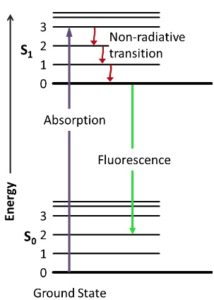
 Molecules have many types of energy levels and all of them can be excited by absorbing photons. Two common energy levels are electronic, where the electrons get excited into higher energy levels, and vibrational, where molecules get excited into different types of vibrations. The energy difference in electronic levels is usually in the UV and/or visible, while the energy difference in vibrational levels is usually in the IR, which is much lower in energy. This means that, within each electronic level, there can be a ground vibrational state and several excited vibrational states. this is represented schematically on the left. S0 is the ground electronic state and it has six vibrational states, represented by 0, 1, 2, … . S1 is the excited electronic state and also has six vibrational states. When a molecule absorbs a photon and gets excited to a higher electronic state, it can also be excited into a higher vibrational state. The molecule can then relax into the ground vibrational state of the excited electronic state (S1-0) without emitting radiation. Now, when the molecule relaxes to the ground electronic state, it emits a photon of lower energy in any direction. This is called fluorescence. In the schematic, a UV photon (represented as purple) was absorbed and a green photon was emitted. “But which vibrational state in the ground electronic state does the molecule relax to?” In the schematic, it relaxed to state 2, but any state is possible. In fact, in an experiment where many molecules are excited, all possible relaxations will happen. This creates a fluorescence spectrum that can be used for identification.
Molecules have many types of energy levels and all of them can be excited by absorbing photons. Two common energy levels are electronic, where the electrons get excited into higher energy levels, and vibrational, where molecules get excited into different types of vibrations. The energy difference in electronic levels is usually in the UV and/or visible, while the energy difference in vibrational levels is usually in the IR, which is much lower in energy. This means that, within each electronic level, there can be a ground vibrational state and several excited vibrational states. this is represented schematically on the left. S0 is the ground electronic state and it has six vibrational states, represented by 0, 1, 2, … . S1 is the excited electronic state and also has six vibrational states. When a molecule absorbs a photon and gets excited to a higher electronic state, it can also be excited into a higher vibrational state. The molecule can then relax into the ground vibrational state of the excited electronic state (S1-0) without emitting radiation. Now, when the molecule relaxes to the ground electronic state, it emits a photon of lower energy in any direction. This is called fluorescence. In the schematic, a UV photon (represented as purple) was absorbed and a green photon was emitted. “But which vibrational state in the ground electronic state does the molecule relax to?” In the schematic, it relaxed to state 2, but any state is possible. In fact, in an experiment where many molecules are excited, all possible relaxations will happen. This creates a fluorescence spectrum that can be used for identification.
Household Demonstration: If you have a ~405 nm laser pointer, shining it through olive oil will cause the oil to fluoresce red.





