JG Simmons, ME Reish, JV Foreman, J Liu, H Everitt – Journal of Materials Chemistry …, 2017
The mechanism for producing efficient white light phosphors from sulfidated zinc oxide (ZnO) powders is elucidated. ZnO powders prepared by vacuum annealing produce powders of oxygen-deficient ZnO:Zn, while ZnO powders annealed in a sulfur atmosphere produce a doped ZnO core with a radially-increasing sulfur concentration gradient capped by a shell of zinc sulfide (ZnS) domains, ZnO:S/ZnS. As compared to ZnO:Zn powders, the intensity and quantum efficiency of the broad, green-tinted white defect fluorescence more than doubled for the ZnO:S/ZnS powders. This fluorescence is mediated by certain neutral donor-bound excitons (DBEs), and it is found that the DBE lifetime and the rate of energy transfer to the defect emission band increases for the ZnO:S/ZnS powders. These DBEs are destroyed by photoexcited free holes, and the hypothesis that they are removed by the type-II band alignment of the ZnS cap with the ZnO:S core is confirmed when ZnO:Zn and ZnO:S/ZnS powders under vacuum are dosed with the hole scavenger methanol: defect emission increases as the free hole concentration decreases. The highest ZnO:S/ZnS quantum efficiency occurs when excited through an impurity band, also produced by sulfur doping, whose energy coincides with the light emitting diodes used for commercial solid state lighting.
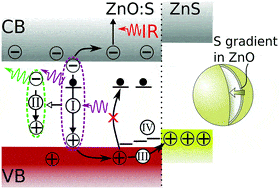
… Simultaneous IR and PL measurements with methanol dosing were performed in a vacuum system with a base pressure of 1×10-9 Torr using a Perkin Elmer Spectrum 100 FTIR for IR collection, and a StellarNet Silver Nova spectrometer was used to collect PL excited by the …





