NHC-Gold (I) and (III) Complexes for Use in Energy Storage and Aryl Halogenation -2012
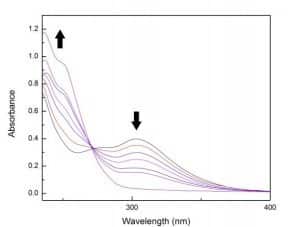
Figure 2.2: UV-Vis time-course spectra for the photochemical reduction of AuCl3(IPr) to AuCl(IPr) in dichloromethane (1.0 cm cuvette) over 2 hours.
N-Heterocyclic Carbene (NHC) complexes of gold(I) and gold(III) of the form AuX(IPr) and AuXCl2(IPr) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene, X = halide or aryl) were prepared and investigated for photochemical activity. Where X = Cl, it was found that an energetically downhill oxidative addition could add an equivalent of Cl2 which could then be subsequently photoreductively eliminated in an energetically uphill step, representing a model energy storage-and-release scheme for chemical fuel formation. Where X = aryl (fluorinated phenyl derivatives), oxidative addition of Cl2 and subsequent reductive elimination produced the gold(I) complex AuCl(IPr) and the chlorinated aryl ligand. The reaction pathway was strongly dependent upon the electronics of the aryl ligand; more electron-rich ligands (X = phenyl) preferentially underwent thermal reductive elimination, while electron-poor ligands (X = pentafluorophenyl) could only be actuated photochemically. A gradient between these extremes was observed in intermediate ligands (X = 4-fluorophenyl, 2,4,6-trifluorophenyl, and 2,3,5,6-tetrafluorophenyl) … Solvents were used directly from commercial sources (VWR) without further purification. UV-vis spectra were recorded on a StellarNet fiber optic UV-Vis … Spectra were recorded on a StellarNet fiber optic UV-Vis spectrophotometer between 190 nm and 1078.5 nm with a 1.0 cm.





