Modern technological advances have made our lives easier – from pharmaceuticals to help us stay healthy, to pesticides that allow greater crop yield – at least in the short term. But, what happens to these compounds once we are done using them? Many of them end up in wastewater streams where they persist for significant amounts of time. My mother always taught me to clean up after myself when I make a mess. Here’s a sampling of StellarNet customers whose mothers must have been similar to mine. Check out how they are working to clean up the environmental mess we’ve created.
Municipal wastewater treatment
 Wastewater “is any water that has been adversely affected in quality by anthropogenic influence,” according to Wikipedia. This includes the various sewage water lines, but also includes less apparent sources, such as runoff and floods that carry off contaminants on the ground. Municipal wastewater (also called domestic sewage) comes from homes, restaurants, schools, hotels, etc. It is further divided into blackwater and graywater. Blackwater comes from toilets, dishwashers, and food preparation sinks; basically, any source of pathogens, like feces, urine, and raw foods. Graywater comes from non-pathogenic sources, like bathroom sinks, laundry machines, and spas. Both types can contain other contaminates, such as lead, pharmaceuticals, or pesticides.
Wastewater “is any water that has been adversely affected in quality by anthropogenic influence,” according to Wikipedia. This includes the various sewage water lines, but also includes less apparent sources, such as runoff and floods that carry off contaminants on the ground. Municipal wastewater (also called domestic sewage) comes from homes, restaurants, schools, hotels, etc. It is further divided into blackwater and graywater. Blackwater comes from toilets, dishwashers, and food preparation sinks; basically, any source of pathogens, like feces, urine, and raw foods. Graywater comes from non-pathogenic sources, like bathroom sinks, laundry machines, and spas. Both types can contain other contaminates, such as lead, pharmaceuticals, or pesticides.
The Boyd group, now at Erskine College, and the Marugan group at Universidad Rey Juan Carlos have developed devices for treating blackwater. Loetscher et. al. made a coiled reactor out of acrylic and deposited TiO2 on the surfaces [1]. TiO2 is a photocatalyst, meaning that light can cause it to catalyze reactions. In this case, light (specifically UV light) causes electron-hole pairs in TiO2 to separate, which generates secondary radicals to degrade pollutants. In the coil reactor, the UV light source was put in the center of the coil. A schematic of the device is shown below.
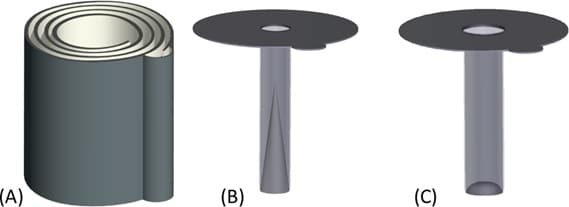
Reprinted with permission from Ref. [1]. Copyright 2009 American Chemical Society.

The Marugan group took a different approach. They built a solar simulator device, characterized by a BLUE-Wave spectrometer, and used it to illuminate a flow cell apparatus [2]. They also used TiO2 to catalyze degradation of E. coli, pharmaceuticals, and pesticides. At low concentration, the device achieved > 80% degradation of the pollutants and bacteria were inactivated by 5 logarithmic units.
At the University of California, Berkeley, the Gutierrez, Lee, and Hermanowicz groups have joined forces in a project called Solar Optics-based Active Pasteurization for Greywater Reuse and Integrated Thermal Building Control, or SOAP For GRIT (scientists do love their acronyms). Vivek Rao, a student in the Hermanowicz group, has this to say about the project:
Agricultural wastewater
When water runs off crop fields, it brings with it all the pesticides that have been applied to the crops. The Cullen group at the Dublin  Institute of Technology decided to apply their atmospheric cold plasma technology to pesticide degradation [3]. Plasma discharges generate radical oxygen and nitrogen species that can degrade many things, including cancer cells and pesticides. In this experiment, a BLACK-Comet was used to measure the emission spectrum of the plasma, confirming the reactive species. A discharge formed from 80kV applied for 8 minutes was able to degrade 79% of dichlorvos, 70% of melathion, and 58% of endosulfan, three common pesticides. The degradation products were also less toxic.
Institute of Technology decided to apply their atmospheric cold plasma technology to pesticide degradation [3]. Plasma discharges generate radical oxygen and nitrogen species that can degrade many things, including cancer cells and pesticides. In this experiment, a BLACK-Comet was used to measure the emission spectrum of the plasma, confirming the reactive species. A discharge formed from 80kV applied for 8 minutes was able to degrade 79% of dichlorvos, 70% of melathion, and 58% of endosulfan, three common pesticides. The degradation products were also less toxic.
This is an encouraging result for three pesticides, but there are a plethora of other pesticides available. Many of them are phenolic compounds, so researchers often study simple phenols as model compounds for other pesticides. The Gang group at the University of Louisiana at Lafayette managed to synthesize an ordered mesoporous carbon (OMC) material for adsorbing resorcinol [4]. They synthesized the OMCs from Santa Barbara Amorphous type material (a mesoporous silica nanoparticle) and tested several modifications to the OMCs, such as using a crosslinking agent to add nitrogen-containing functional groups. A StellarNet Raman system was used to characterize the specific functionalizations. They found that the crosslinking agent greatly enhanced the adsorption of the OMCs, achieving 37% more adsorption than the standard activated carbon.
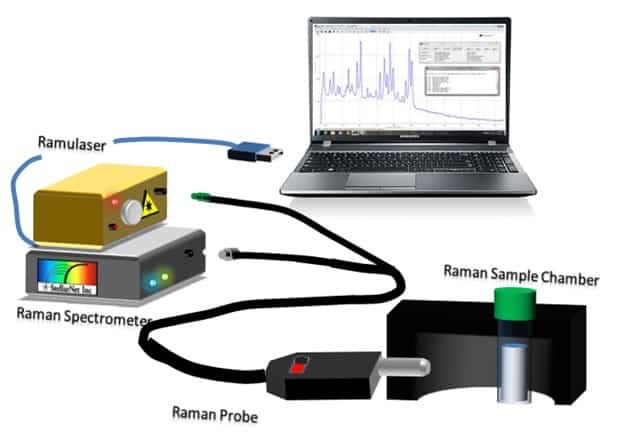
StellarNet Raman Spectrometer System consisting of Ramulaser, Raman-HR-TEC spectrometer, & Raman Probe
Researchers at Lakehead University even filed a patent based on their device [5]. They made a bifunctional electrode with a conductive substrate coated with a photocatalyst and electrocatalyst. Their proof of concept used Ti as the substrate, TiO2 as the photocatalyst, and Ta2O2-IrO2 as the electrocatalyst. When a bias is applied to the device, the electrocatalyst oxidizes a first pollutant. This causes a potential drop at the photocatalyst. The products from the electrocatalyst are also available to scavenge electrons. These two processes, plus the initial bias, inhibit recombination at the photocatalyst after it has been illuminated. This causes a second pollutant to be oxidized at the photocatalyst. The inventors tested the device on 4nitrophenol and 2-nitrophenol, using a BLUE-Wave spectrometer to monitor the degradation with UV-vis spectroscopy. Using the bifunctional electrode was 10-100x faster than using just the electrocatalyst or photocatalyst!
While it may seem like there’s a never ending list of potential contaminants in the environment, these results suggest that they can be cleaned up with the same general methods. Most notably, a majority of the studies mentioned here used TiO2, which seems to be the wonder material of choice for decontamination. Modern technology made this mess, but modern technology can also clean it up.
References
1. Loetscher LH, Carey JM, Skiles SL, Carey VM, Boyd JE. Titania−Acrylic Coil Reactor for Photocatalytic Water Purification and Sterilization. Ind Eng Chem Res. 2009;48: 4697– 4702.
2. Philippe KK, Timmers R, van Grieken R, Marugan J. Photocatalytic Disinfection and Removal of Emerging Pollutants from Effluents of Biological Wastewater Treatments, Using a Newly Developed Large-Scale Solar Simulator. Ind Eng Chem Res. 2016;55: 2952–2958.
3. Sarangapani C, Misra NN, Milosavljevic V, Bourke P, O’Regan F, Cullen PJ. Pesticide degradation in water using atmospheric air cold plasma. Journal of Water Process Engineering. 2016;9: 225–232.
4. Shou W, Chao B, Ahmad ZU, Gang DD. Ordered mesoporous carbon preparation by the in situ radical polymerization of acrylamide and its application for resorcinol removal. J Appl Polym Sci. 2016; doi:10.1002/app.43426
5. Chen A, Asmussen RM, Tian M. Method and system for combined photocatalytic and electrochemical wastewater remediation [Internet]. US Patent. 20120279872:A1, 2012. Available: https://www.google.com/patents/US20120279872

By Debra McCaffrey, PhD
StellarNet Technical Staff Writer
UC Berkeley Chemistry





